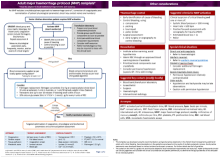
The Patient blood management guideline for adults with critical bleeding (guideline) is an update of the Patient Blood Management Guidelines: Module 1 Critical Bleeding/ Massive Transfusion (2011).
The intent of the guideline is to provide updated clinical guidance for health professionals providing immediate in-hospital care for adults with critical bleeding, including the use of a major haemorrhage protocol (MHP) to guide the use of blood components, blood products and blood conservation strategies.
The clinical guidance is based on scientific evidence and consensus among clinical experts. The NBA gratefully acknowledges the dedication and expertise provided by the multidisciplinary Clinical/Consumer Reference Group.
The guideline was published in electronic format and on MAGICapp, a web-based platform for guidelines on 10 August 2023.
The guideline and accompanying resources can be accessed by clicking on the links below.
Patient blood management guideline for adults with critical bleeding
On MAGICapp
![]() PDF created from MAGICapp
PDF created from MAGICapp
Please note this guideline is maintained on MAGICapp. The electronic version on MAGICapp and PDF version on this website are the approved version and most current. Any downloaded or printed version however, is only current as at the day of download/printing. Any updates to the guideline will be acknowledged on this webpage. To keep up to date with the latest changes to this guideline click on subscribe in MAGICapp.
If you would like to keep up to date with all our guidelines and receive patient blood management updates you can subscribe to the PBM mailing list through BloodPortal.
Major Haemorrhage Protocol (MHP) template
An editable template to be adapted to local institutional requirements and resources.
Technical report on the methodology and findings of the evidence review process

Volume 1 – Description of the methodology and results of the evidence review process
Volume 2 – Literature searches and critical appraisal of the studies
Volume 3 – Data extraction forms
Summary of clinical guidance
The Quick Reference Guide comprises a:
-
summary of the recommendations (Rs) developed by the CRG, based on a systematic review of evidence
-
summary of the good practice statements (GPSs) that were developed by the CRG through an expert consensus process
-
MHP template which is designed to be adapted to mee local needs and resources
To print the A5 format, print double-sided on the ‘short edge’ and fold the two pages into a booklet.
College and Society endorsements
The NBA greatly appreciates and acknowledges the input received from the clinical community in producing the guideline. We are pleased to acknowledge that the following organisations have formally endorsed the guideline:
-
Australian & New Zealand Society of Blood Transfusion
For more information
To provide feedback and inform future reviews of this guideline, please send any comments on its content or implementation, or on the accompanying materials, to the project manager at:
-
Email guidelines@blood.gov.au
-
Mail
Patient Blood Management Guidelines
National Blood Authority
Locked Bag 8430
Canberra ACT 2601